Abstract
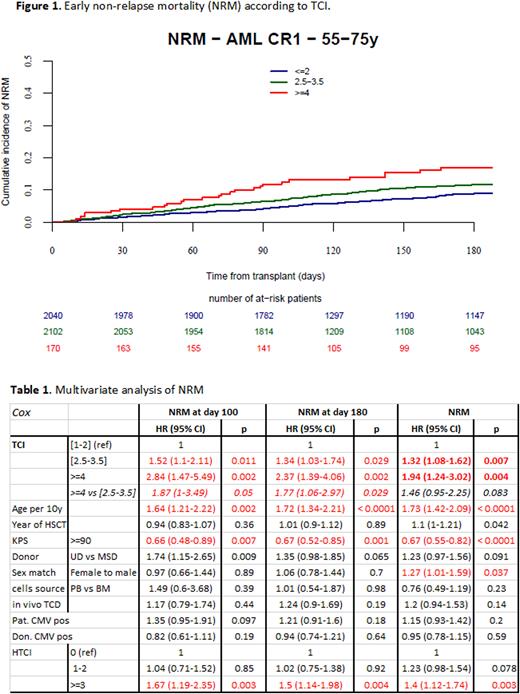
Introduction: Since non-relapse mortality (NRM) after allogeneic hematopoietic cell transplantation (HCT) is influenced by the conditioning regimen, it is essential to precisely calculate its intensity for any patient. Therefore, the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT) registry previously developed the Transplant Conditioning Intensity (TCI) score to define conditioning regimens based on the sum of the doses (weight scores) for every component of the pre-transplantation regimen. HCT patients (pts) were stratified by a 3-index scheme with a low [1-2], intermediate [2.5-3.5] or high [4-6] TCI-score. (Bone Marrow Transplantation 2020; 55:1114) When tested in a large registry dataset of acute myeloid leukemia (AML) pts >45 years of age, TCI grouping was highly predictive for early and 2-year NRM, and better compared with the classical 2-index reduced intensity conditioning/ myeloablative conditioning (RIC/MAC) classification. The aim of the current study was to validate using a distinct dataset, the ability of the original TCI classification scheme to predict post-HCT NRM. Methods: TCI score was applied to a timely distinct cohort reported to the EBMT as the one used to establish the TCI classification scheme. We tested the impact of TCI in predicting early (day 180) NRM (defined as any death without previous relapse) in 55-75 years of age AML first complete remission (CR-1) pts who were transplanted between 2018 and 2021. Included pts were allografted with peripheral blood stem cells (PBSC, 96.4%) or bone marrow (BM, 3.6%) from a matched sibling donor (MSD, 25%) or unrelated donor (UD, 75%). NRM at 2 years was a secondary endpoint. Results: 4,312 AML pts (median age 63.4, interquartile range 59.5-67.2) were assigned to a TCI category, as previously described. 49% of the pts received a low [1-2], 47% an intermediate [2.5-3.5] but only 4% of the pts received a high [4-6] TCI-score regimen. As expected, there was an inverse relationship between age, Karnofsky status and hematopoietic cell transplant-specific comorbidity index (HCT-CI) and TCI group (p<0.0001). Besides a higher prevalence of MSD transplantations in the high (40%) as compared to low (21.6%) and intermediate (26.4%) TCI-groups (p<0.0001), pts were well balanced within TCI groups regarding other transplant and patient characteristics. The day (d)100, d180 and 2-year NRM ranged from 4.8-12.5%, 8.9-17%, and 17.4-23.9% between low, intemediate and high TCI scores, respectively. In univariate analysis, TCI provided a highly significant risk stratification for early and 2-year NRM (p<0.0001 for all pairwise comparisons between groups) (Figure 1). In the multivariate Cox regression analysis adjusted for age, Karnofsky status, HCT-CI and other variables, there was a significant increase in risk of early NRM with each TCI group (Table 1). Compared to low TCI, pts receiving intermediate and high score TCI regimens had a significantly increased risk for d180 NRM (hazard ratio [HR] 1.34, 95% CI, 1.03-1.74, p=0.029 and HR 2.37, 1.39-4.06, p<0.002, respectively). The HRs for d180 NRM pts receiving a high TCI [4-6] score regimen was 1.77 (1.06-2.97, p=0.029) when compared to the intermediate TCI [2.5-3.5] group. As expected, early NRM was also significantly influenced by incremental age, Karnofsky status score <90 and HCT-CI >=3. TCI grouping was also significantly associated with overall NRM. Similar results were found when the analysis was restricted to pts aged between 55 and 65 years (N=2,640). There was good concordance between RIC/MAC and low/high TCI score grouping, whereas 49% of the MAC pts and 51% of the RIC pts were re-classified as having received a reduced toxicity conditioning regimen with a TCI score of 2.5-3.5. Conclusion: Here, we validated in an independent patient cohort, the recently proposed 3-group TCI score for its ability to predict early NRM in AML pts >55 years of age. TCI predicts early NRM independently of other known prognostic factors such as age, Karnofsky, or HCT-CI. Compared to the current RIC/MAC classification model, the TCI provides a finer and more precise tool which may be easily calculated for any patient to offer safer transplantation especially in older and unfit pts. TCI may be used as a new, well-defined, and standard terminology for conditioning intensity in registry databases, in the literature and in clinical trials.
Disclosures
Spyridonidis:Stemline/Menarini: Consultancy. Labopin:Jazz Pharmaceuticals: Honoraria. Craddock:Novartis: Consultancy; Abbvie: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; JAZZ: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy. Wagner:Amgen: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other: NA; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medac: Other: NA; Novartis: Membership on an entity's Board of Directors or advisory committees. Dreger:AbbVie, AstraZeneca, Gilead, Novartis, Riemser, and Roche: Speakers Bureau; AbbVie, AstraZeneca, Bluebird Bio, Gilead, Janssen, Novartis, Riemser, and Roche: Consultancy. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Forcade:Sanofi: Other: Travel Support; MSD: Other: Travel Support; Novartis: Speakers Bureau; Jazz: Other: Travel Support, Speakers Bureau; GSK: Speakers Bureau; Gilead: Other: Travel Support, Speakers Bureau. Ciceri:Kite Pharma: Consultancy. Mohty:Jazz Pharmaceuticals: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal